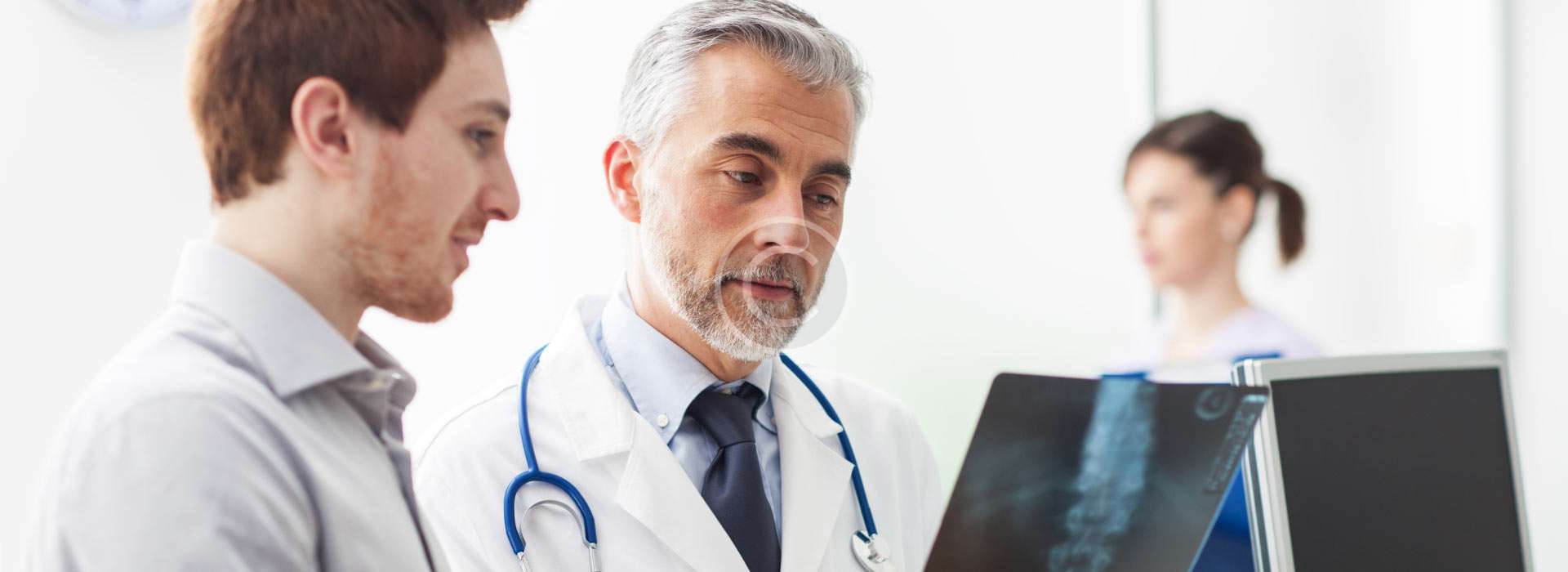
Simplifying Market Entry into Libya
Al Samiya Company provides hands-on support for pharmaceutical manufacturers and wholesalers aiming to enter the Libyan market. We assist in navigating the complex regulatory landscape, ensuring all documentation and registration requirements are met with precision and efficiency.
Expertise in Local Regulations
With a deep understanding of Libya’s Ministry of Health guidelines, we help streamline the product registration process—from dossier preparation and submission to regulatory follow-up. Our team ensures compliance while minimizing delays, making your products market-ready faster.
Comprehensive Documentation Support
We provide full assistance with the preparation and handling of technical documents, Certificates of Analysis (CoA), Free Sale Certificates, GMP documentation, and more. Our experience ensures nothing is overlooked, whether it’s for registration or shipment clearance.
Partnering for Long-Term Success
By managing the administrative and regulatory burdens on your behalf, Al Samiya becomes more than a distributor—we become your local compliance partner. Our trusted track record gives international suppliers the confidence to grow in the Libyan market without bureaucratic friction.